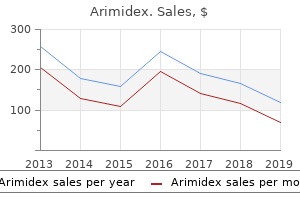
Buy arimidex 1 mg
The Liver Is Responsible for the Uptake of Remnant Lipoproteins Chylomicron remnants are taken up by the liver by receptor-mediated endocytosis, and the cholesteryl esters and triacylglycerols are hydrolyzed and metabolized. Hepatic lipase has a dual position: (1) in performing as a ligand to the lipoprotein and (2) in hydrolyzing its triacylglycerol and phospholipid. Only one molecule of apo B-100 is current in each of these lipoprotein particles, and that is conserved in the course of the transformations. It appears plasma lipoproteins are interrelated parts of one or more of} metabolic cycles that collectively are responsible for the advanced means of plasma lipid transport. The fatty acids used are derived from two possible sources: (1) synthesis within the liver from acetyl-CoA derived mainly from carbohydrate (perhaps not so necessary in humans) and (2) uptake of free fatty acids from the circulation. The first supply is predominant in the well-fed situation, when fatty acid synthesis is excessive and the extent of circulating free fatty acids is low. Free fatty acids from the circulation are the main supply during starvation, the feeding of high-fat diets, or in diabetes mellitus, when hepatic lipogenesis is inhibited. Added vitamin E or a supply of selenium has a protecting impact by combating lipid peroxidation. When accumulation of lipid in the liver becomes persistent, fibrotic modifications happen in the cells that progress to cirrhosis and impaired liver perform. The first type is related to raised levels of plasma free fatty acids ensuing from mobilization of fats from adipose tissue or from the hydrolysis of lipoprotein triacylglycerol by lipoprotein lipase in extrahepatic tissues. In uncontrolled diabetes mellitus, twin lamb disease, and ketosis in cattle, fatty infiltration is sufficiently extreme to cause seen pallor (fatty appearance) and enlargement of the liver with possible liver dysfunction. Theoretically, the lesion could also be} outcome of} (1) a block in apolipoprotein synthesis, (2) a block in the synthesis of the lipoprotein from lipid and apolipoprotein, (3) a failure in provision of phospholipids would possibly be} found in lipoproteins, or (4) a failure in the secretory mechanism itself. One type of fatty liver that has been studied extensively in rats is because of|as a end result of} of} a deficiency of choline, which has due to this fact been referred to as a lipotropic factor. The action of carbon tetrachloride probably entails formation of free radicals Ethanol Also Causes Fatty Liver Alcoholism results in fats accumulation in the liver, hyperlipidemia, and finally cirrhosis. Ethanol consumption over an extended period results in the accumulation of fatty acids in the liver would possibly be} derived from endogenous synthesis rather than from increased mobilization from adipose tissue. The internet impact of inhibiting fatty acid oxidation is to cause increased esterification of fatty acids in triacylglycerol, ensuing in the fatty liver. Oxidation of ethanol results in the formation of acetaldehyde, which is oxidized by aldehyde dehydrogenase, producing acetate. Other effects of ethanol may include increased lipogenesis and cholesterol synthesis from acetyl-CoA, and lipid peroxidation. During translation of apo B-100, microsomal transfer protein-mediated lipid transport permits lipid to turn out to be related to the nascent polypeptide chain. Ethanol may even inhibit the metabolism of some medication, eg, barbiturates, by competing for cytochrome P450-dependent enzymes. This permits the processes of esterification or lipolysis to be regulated individually by many nutritional, metabolic, and hormonal components. The resultant of these two processes determines the magnitude of the free fatty acid pool in adipose tissue, which in turn determines the extent of free fatty acids circulating in the plasma. Since the latter has most profound effects upon the metabolism of other tissues, significantly liver and muscle, the components operating in adipose tissue that regulate the outflow of free fatty acids exert an affect far beyond the tissue itself. Triacylglycerol undergoes hydrolysis by a hormonesensitive lipase to type free fatty acids and glycerol. This lipase is distinct from lipoprotein lipase that catalyzes lipoprotein triacylglycerol hydrolysis earlier than its uptake into extrahepatic tissues (see above). Details of the formation of glycerol 3-phosphate from intermediates of glycolysis are shown in Figure 24�2. A principal action of insulin in adipose tissue is to inhibit the activity of hormone-sensitive lipase, reducing the discharge not only of free fatty acids but of glycerol as nicely. Adipose tissue is rather more delicate to insulin than are many other tissues, which points to adipose tissue as a serious site of insulin action in vivo. Several Hormones Promote Lipolysis Other hormones accelerate the discharge of free fatty acids from adipose tissue and raise the plasma free fatty acid concentration by increasing the speed of lipolysis of the triacylglycerol stores (Figure 25�8). For an optimum impact, most of these lipolytic processes require the presence of glucocorticoids and thyroid hormones. These hormones act in a facilitatory or permissive capability with respect to other lipolytic endocrine components. The mechanism is analogous to that responsible for hormonal stimulation of glycogenolysis (Chapter 18). Insulin additionally stimulates phosphodiesterase and the lipase phosphatase that inactivates hormone-sensitive lipase. The just lately found physique weight regulatory hormone, leptin, stimulates Increased Glucose Metabolism Reduces the Output of Free Fatty Acids When the utilization of glucose by adipose tissue is increased, the free fatty acid outflow decreases. The impact is because of|as a end result of} of} the provision of glycerol 3-phosphate, which enhances esterification of free fatty acids. However, as whole glucose utilization decreases, the larger proportion of the glucose is directed to the formation of glycerol 3-phosphate for the esterification of acyl-CoA, which helps to minimize the efflux of free fatty acids. Insulin additionally will increase the activity of pyruvate dehydrogenase, acetylCoA carboxylase, and glycerol phosphate acyltransferase, reinforcing the consequences of increased glucose uptake on the enhancement of fatty acid and acylglycerol synthesis. Positive (+) and unfavorable (-) regulatory effects are represented by damaged lines and substrate flow by solid lines. Human adipose tissue is unresponsive to many of the lipolytic hormones apart from the catecholamines. On consideration of the profound derangement of metabolism in diabetes mellitus (due massive part|largely} to increased release of free fatty acids from the depots) and reality that|the fact that} insulin to a large extent corrects the condi- A Variety of Mechanisms Have Evolved for Fine Control of Adipose Tissue Metabolism Human adipose tissue an necessary site of lipogenesis. Activity of the respiratory chain produces warmth along with translocating protons (Chapter 12). The passage of H+ via thermogenin is inhibited by purine nucleotides when brown adipose tissue is unstimulated. Holm C et al: Molecular mechanisms regulating hormone delicate lipase and lipolysis. It is synthesized in many of} tissues from acetyl-CoA and is the precursor of all other steroids in the physique corresponding to corticosteroids, sex hormones, bile acids, and vitamin D. Step 3-Six Isoprenoid Units Form Squalene: Isopentenyl diphosphate is isomerized by a shift of the double bond to type dimethylallyl diphosphate, then condensed with another molecule of isopentenyl diphosphate to type the ten-carbon intermediate geranyl diphosphate (Figure 26�2). Two molecules of farnesyl diphosphate condense at the diphosphate finish to type squalene. Acetyl-CoA Is the Source of All Carbon Atoms in Cholesterol the biosynthesis of cholesterol could also be} divided into 5 steps: (1) Synthesis of mevalonate happens from acetylCoA (Figure 26�1). Protein prenylation is believed to facilitate the anchoring of proteins into lipoid membranes and may be concerned in protein-protein interactions and membraneassociated protein trafficking. In addition to these mechanisms regulating the speed of protein synthesis, the enzyme activity modulated more rapidly by posttranslational modification (Figure 26�4). The open and solid circles point out the fate of every of the carbons in the acetyl moiety of acetylCoA. The methyl group on C14 is transferred to C13 and that on C8 to C14 as cyclization happens, catalyzed by oxidosqualene:lanosterol cyclase. Step 5-Formation of Cholesterol: the formation of cholesterol from lanosterol takes place in the membranes of the endoplasmic reticulum and entails modifications in the steroid nucleus and side chain (Figure 26�3). The methyl groups on C14 and C4 are removed to type 14-desmethyl lanosterol and then zymosterol. The double bond at C8�C9 is subsequently moved to C5�C6 in two steps, forming desmosterol. Biosynthesis of squalene, ubiquinone, dolichol, and other polyisoprene derivatives. The numbered positions are those of the steroid nucleus and the open and solid circles point out the fate of every of the carbons in the acetyl moiety of acetyl-CoA. The apoprotein and cholesteryl ester are then hydrolyzed in the lysosomes, and cholesterol is translocated into the cell. Of the cholesterol absorbed, 80�90% is esterified with long-chain fatty acids in the intestinal mucosa.
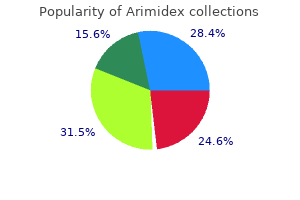
Generic arimidex 1 mg
DeoxyHbS polymerizes at low O2 concentrations, forming fibers that distort erythrocytes into sickle shapes. Alpha and beta thalassemias are anemias that outcome from decreased production of and subunits of HbA, respectively. Mario N, Baudin B, Giboudeau J: Qualitative and quantitative evaluation of hemoglobin variants by capillary isoelectric focusing. Deficiencies in the amount or catalytic activity of key enzymes finish up} from genetic defects, nutritional deficits, or toxins. The widespread names for most enzymes derive from their most distinctive characteristic: their capability to catalyze a selected chemical response. Unfortunately, while many modifiers name the precise substrate involved (xanthine oxidase), others establish the supply of the enzyme (pancreatic ribonuclease), specify its mode of regulation (hormone-sensitive lipase), or name a distinguishing characteristic of its mechanism (a cysteine protease). Finally, the time period "hexose-6" indicates that the alcohol phosphorylated is that of carbon six of a hexose. Like all catalysts, enzymes are neither consumed nor completely altered as a consequence of their participation in a response. Enzymes are additionally stereospecific catalysts and usually catalyze reactions only of specific stereoisomers of a given compound-for instance, D- however not L-sugars, L- however not D-amino acids. Figure 7�1 illustrates why the enzyme-catalyzed discount of the nonchiral substrate pyruvate produces L-lactate somewhat a racemic combination of D- and L-lactate. Planar representation of the "threepoint attachment" of a substrate to the active site of an enzyme. Although atoms 1 and four are equivalent, quickly as} atoms 2 and three are certain to their complementary websites on the enzyme, only atom 1 can bind. Metal ions that take part in redox reactions usually are complexed to prosthetic groups such as heme (Chapter 6) or ironsulfur clusters (Chapter 12). Metals additionally might facilitate the binding and orientation of substrates, the formation of covalent bonds with response intermediates (Co2+ in coenzyme B12), or interaction with substrates to render them extra electrophilic (electron-poor) or nucleophilic (electron-rich). Transferases catalyze switch of groups such as methyl or glycosyl groups from a donor molecule to an acceptor molecule. Coenzymes Serve as Substrate Shuttles Coenzymes serve as recyclable shuttles-or group switch reagents-that transport many substrates from their point of era to their point of utilization. Many Coenzymes, Cofactors, & Prosthetic Groups Are Derivatives of B Vitamins the water-soluble B vitamins provide essential components of numerous coenzymes. Two-dimensional representation of a dipeptide substrate, glycyl-tyrosine, certain inside the active site of carboxypeptidase A. As its pyrophosphate, thiamin participates in decarboxylation of -keto acids and folic acid and cobamide coenzymes function in one-carbon metabolism. Termed the active site, this setting usually takes the form of a cleft or pocket. The active websites of multimeric enzymes typically are positioned at the interface between subunits and recruit residues from a couple of monomer. The three-dimensional active site both shields substrates from solvent and facilitates catalysis. This simultaneously aligns parts of the substrate that can undergo change with the chemical useful groups of peptidyl aminoacyl residues. Many amino acyl residues drawn from numerous parts of the polypeptide chain (Figure 7�3) con- Catalysis by Proximity For molecules to react, they must to|they have to} come inside bondforming distance of one another other}. When an enzyme binds substrate molecules in its active site, it creates a area of high local substrate focus. This setting additionally orients the substrate molecules spatially in a position perfect for them to interact, leading to price enhancements of a minimum of|no much less than} a thousandfold. The induced fit model has been amply confirmed by biophysical studies of enzyme movement during substrate binding. Reactions whose rates are responsive to all the acids or bases present are said to be subject to basic acid or basic base catalysis. Catalysis by Strain Enzymes that catalyze lytic reactions which contain breaking a covalent bond usually bind their substrates in a conformation slightly unfavorable for the bond that can undergo cleavage. Covalent Catalysis the method of covalent catalysis includes the formation of a covalent bond between the enzyme and a number of} substrates. On completion of the response, the enzyme returns to its original unmodified state. Covalent catalysis is particularly widespread among enzymes that catalyze group switch reactions. Residues on the enzyme that take part in covalent catalysis usually are cysteine or serine and sometimes histidine. Covalent catalysis typically follows a "ping-pong" mechanism-one during which the first substrate is certain and its product released prior to the binding of the second substrate (Figure 7�4). Catalysis includes two conserved aspartyl residues which act as acid-base catalysts. In the first stage of the response, an aspartate functioning as a basic base (Asp X, Figure 7�6) extracts a proton from a water molecule, making it extra nucleophilic. This ensuing nucleophile then attacks the electrophilic carbonyl carbon of the peptide bond focused for hydrolysis, forming a tetrahedral transition state intermediate. Two totally different active site aspartates thus can act simultaneously as a basic base or as a basic acid. This is possible their instant setting favors ionization of 1 however not the opposite. The hydrogen on Asp 102 then shuttles by way of His fifty seven to the amino group liberated when the peptide bond is cleaved. The charge-relay network now prompts the water molecule by withdrawing a proton by way of His fifty seven to Asp 102. While modified in the course of the strategy of catalysis, chymotrypsin emerges unchanged on completion of the response. Trypsin and elastase employ an analogous catalytic mechanism, however the numbers of the residues in their Ser-His-Asp proton shuttles differ. Fructose-2,6-Bisphosphatase Fructose-2,6-bisphosphatase, a regulatory enzyme of gluconeogenesis (Chapter 19), catalyzes the hydrolytic launch of the phosphate on carbon 2 of fructose 2,6bisphosphate. Enzyme households seem to arise by way of gene duplication occasions that create a second copy of the gene which encodes a specific enzyme. The proteins encoded by the 2 genes can then evolve independently to recognize totally different substrates-resulting, for instance, in chymotrypsin, which cleaves peptide bonds on the carboxyl terminal facet of enormous hydrophobic amino acids; and trypsin, which cleaves peptide bonds on the carboxyl terminal facet of basic amino acids. Proteins that share massive quantity of|numerous|a lot of} conserved residues are said to be homologous to each other. Table 7�1 illustrates the first structural conservation of two components of the charge-relay network for serine proteases. Among the most highly conserved residues are those that participate immediately in catalysis. Like the members of other protein households, these protein catalysts or isozymes arise by way of gene duplication. Some isozymes can also improve survival by providing a "backup" copy of an important enzyme. Assays of the catalytic activity of enzymes are regularly used in research and medical laboratories. His 258 four Enzyme-Linked Immunoassays the sensitivity of enzyme assays exploited to detect proteins that lack catalytic activity. When serum or other samples to be tested are positioned in a plastic microtiter plate, the proteins adhere to the plastic surface and are immobilized. Any remaining absorbing areas of the properly are then "blocked" by adding a nonantigenic protein such as bovine serum albumin. The antibodies adhere to the immobilized antigen and these are themselves immobilized. The presence and amount of certain antibody are then decided by adding the substrate for the reporter enzyme. Amino acid sequences in the neighborhood of the catalytic websites of bovine proteases. Regions shown are these on either facet of the catalytic site seryl (S) and histidyl (H) residues. An various strategy is to devise an artificial substrate whose product absorbs gentle. However, following hydrolysis, the ensuing p-nitrophenylate anion absorbs gentle at 419 nm.
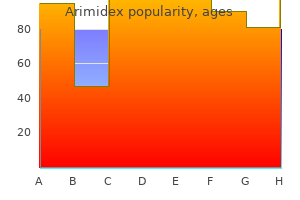
Best arimidex 1 mg
All copies must retain all creator credits and copyright notices included in the original doc. Under no circumstances is it permissible to sell or distribute on a industrial basis, or to claim authorship of, copies of material reproduced from this publication. Except as expressly offered above, no a part of} this publication could also be} reproduced or transmitted in any kind or by any means, digital or mechanical, including photocopying, recording, or by any data storage and retrieval system, with out written permission of the creator or authors. This materials is intended for educational use solely by training well being care employees or students and faculty in a well being care subject. It has been recognized lots of of years} that abnormalities in urine might point out illness and the analysis of urine are developed from simple visible examination to the modern automated methods. The need for trained human resources in the subject is, subsequently, very important not just for affected person care but also for preventive measures. Authors from greater well being educating institutions are those that compiled the lecture note. Books, and existing lecture manuscripts have been mainly used to develop this primary draft of lecture materials. Useful ideas of different instructors of the course have been also incorporated to standardize it to the current standing. The authors hope to enhance the draft through additional consultations, pretests and revisions. Special thanks are prolonged to Professor Dennis Carlson for growing lecture notes and for his technical and ethical help. The writers also express their special thanks and gratitude to Ato Aklilu Mulugeta of the Administrative and Finance Service, the Carter Center for his materials and logistic help. Finally we thank all individuals and institutions which have in some contributed to this lecture note. Perhaps, one of the earliest recognized record of urine test was the strategy of pouring urine on the bottom and observing whether or not or not it attracted bugs. The attraction of bugs indicates " honey urine " which was recognized to be excreted by folks with boils. Today checking sugar in urine is a test to detect diabetes (And untreated diabetes still undergo from boils). It used to detect intrinsic situations that may adversely affect on} the kidneys or urinary tract. Consequently, substances usually retained by a kidney or excreted in small quantities might appear in the urine in large portions, or substances usually excreted could also be} retained by kidney. It also helps the professionals to understand and students acquire properly as|in addition to} different well being the required procedures, that are helpful in the investigation of normal and abnormal urine constituents and interpretation of the results. Introduction the Renal System is a system which consists of two kidneys, two ureters, one bladder and one urethra. As the elements of the renal system the kidneys have the next capabilities: Regulation of water and electrolyte (such as chloride, potassium, calcium, hydrogen, magnessium, and phosphate ions) balances. The kidneys are the first means for the eliminating waste merchandise of physique metabolism which might be} not needed by the physique. These merchandise embrace urea from the metabolism of amino acids, uric acid from the nucleic acids, creatinine from muscle creatine, bilirubin from the breakdown of hemoglobin. The kidneys synthesize glucose from amino acids and different precursors, like lactate and glycerol, during extended fasting by the process known as gluconeogenesis. Each human kidney weighs a hundred and fifty gms and measures 1x2x3 inches (thickness, width, and length). A coronal section of the kidney exhibits an outer reddish granular layer known as renal medulla. In the renal medulla the triangular and wedge shaped construction renal pyramids. The ideas of the pyramids discovered on the renal papillae at which urine is drained into cavities Renal Calyces. Renal Calyces drain urine into renal pelvis, then to ureter, which in flip drain to bladder and then through the urethra is voided out. Each nephron consists of a glomerulus, which is essentially filtering system, and a tubule through which the filtered liquid passes. The afferent arteriole, which carries blood from the renal artery into the glomerulus divides to kind a capillary community. These capillaries re-unite to kind the efferent arteriole, through which blood leaves the glomerulus. The blood vessels thus comply with the course of the tubule, forming a surrounding capillary community. The tubular portion of every nephron has several of} distinct structural and practical segments. The uppermost portion, which steady with the glomerulus, is the proximal convoluted tubule, followed by the skinny walled segment and the distal convoluted tubule respectively. The descending limb of the proximal tubule (the thin-walled segment) and the distal tubule kind a loop the Loop of Henle. The distal convoluted tubules from several of} nephrons drain right into a four collecting tubule. A variety of these collecting tubules kind the the collecting ducts then join together to kind the papillary ducts. The latter empty at the ideas of the papillae into the calyces, which in flip drain into the renal pelvis. The blood enters the glomerulus of every nephron by passing through the afferent arteriole into the glomerular capillaries. The capillary partitions in the glomerulus are highly permeable to water and the low molecular-weight elements of the plasma. Many elements of the plasma filtrate such as glucose, water, and amino acids, are partially or fully reabsorbed by the capillaries surrounding the proximal tubules. In the distal tubules, extra water is reabsorbed and potassium and hydrogen ions are secreted. The Loop of Henle and the system of collecting tubules are the principal sites where the urine is concentrated as a mechanism for conserving physique water. Urine fashioned by the three physiological processes which might be} by glomerular filtration, tubular reabsorption, and tubular secretion, is collected by the collecting duct and passes into bladder through ureters and then comes out through urethra. Clearance is often defined because the blood volume that incorporates the amount of a substance excreted in the urine per minute. Hence creatinine clearance is used clinically to give an approximate indication of glomerular filtrate fee and, subsequently, as a test of kidney operate. When the filtration fee falls, the concentration of creatinine in the plasma rises. The creatinine clearance test express the quantity of blood containing the amount of creatinine excreted by the kidney in one minute. Creatinine is then decided in both urine and serum, and the creatinine clearance calculated in milliliters per minute (ml / minute) Ccr ml / minute = U x V S Where U= Urine Creatinine Concentration in mol/l V= Volume of urine in ml per 24 hrs S= Serum Creatinine Concentration in mol/l Normal Range: the traditional Ccr value often ranges between 110 � one hundred forty ml / minute. Urea and creatinine, nevertheless, are all the time current in the urine independent of the blood degree as a result of|as a result of} little or no, if any, of these substance is reabsorbed. Mention the factors that determine the selective passage of molecules through the glomerular membrane. Identify the commonly used preservatives and know the benefits and drawbacks of their use. Improper assortment might invalidate the results of the laboratory procedures, regardless of how fastidiously and elegantly the exams are carried out. Before specimens are collected, the containers must be cleaned and thoroughly dried. Disposable containers of plastic or coated paper can be found in lots of} sizes and are supplied with lids to cut back bacterial and different forms of contamination. The system consists of a flat-bottomed paper assortment cup, a 15 ml 10 plastic tube with a plastic snap-cap and self-adhesive identification label. After the label is filled out and attached, the specimen is ready to|is ready to} be transported and analyzed. Large, wide-mouthed plastic or glass containers with screw cap tops are used for cumulative assortment of urine over a protracted period of time.
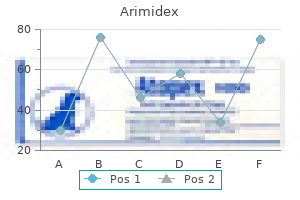
Quality 1 mg arimidex
Pressure during fermentation decreases ester formation in the identical method as the higher alcohols. Higher alcohols and esters in defined concentrations are necessary for the aroma profile of a high-quality beer. Apart from environment friendly trub elimination, an important factor within the formation and reduction of those flavoractive substances is the yeast pressure and the absence of spoiling microorganisms in beer. They can strongly impair beer flavor and are additionally liable for the so-called lightstruck flavor in beer. Glycerol, a multivalent alcohol, is shaped during glycolysis; its concentration is dependent upon by} the quantity of fermented sugars (1300�2000 mg/l) and is due to this fact proportional to the gravity of the wort. Aldehydes and ketones are liable for the aroma of the green beer and for the stale flavor. Acetaldehyde is shaped within the green beer through the first three days and provides beer an unripe, unbalanced taste. Since it vanishes in later fermentation phases, it presents no technological difficulties. In the green beer section the acetaldehyde content material is about 20�40 mg/l; within the finished beer values of eight to 10 mg/l are found. Off-flavors in beer are usually brought on by excessive levels of the vicinal diketones diacetyl and a couple of|and a pair of},3-pentandione. In addition to the formation of byproducts, a number of|numerous|a variety of} different reactions and modifications take place through the fermentation and these are outlined within the following sections. High-molecular-weight polypeptides turn out to be insoluble and are later filtered out of the beer. A rapid fermentation is advantageous for the precipitation of protein�polyphenol complexes. The beer matures quicker, 212 eight Fermentation, Maturation and Storage can be filtered with out problems and has an excellent non-biological stability. The increase of the decreasing potential is intently associated to the uptake of dissolved oxygen by the yeast initially of the fermentation. The redox potential in beer can be measured by the rH worth, the Indicator Time Test or the oxygen content material in beer. The oxygen of the aerated wort is absorbed by the yeast some hours after pitching after which reaches values of zero. Some substances change their shade in accordance with the pH drop; some are adsorbed on the surface of the yeast and are removed with the settling yeast. The yeast cell count should be under 2 million cells/ml after conditioning beer in this method. First, the young beer turns into coated by a skinny white layer of froth � the sign that fermentation has begun. In the following stage of excessive kr�usen, fermentation has entered in its most intensive part. At the tip of fermentation the kr�usen collapses slowly and a skinny brown layer signifies that the yeast has accomplished its work. The main objectives of the fermentation are to reach: � � � � � � Constant fermentation occasions. Therefore, if a concentration above 10 mg/l is reached, it has to be declared on the label. In addition, it influenced by technological parameters during pitching. Lower aeration, excessive unique gravity and poor yeast vitality result in higher sulfur dioxide contents; nonetheless, yeast count at pitching important, as higher extract and decrease oxygen per cell can increase sulfur dioxide [4]. In calculating the total diacetyl concentration, 2acetolactate must be added to the quantity of free diacetyl. During the propagation section the yeast cells need quite a few nitrogen compounds for the formation of yeast protein. This extremely temperature-dependent step is catalyzed by yeast enzymes and occurs very slowly under 10 �C. Diacetyl itself is current in very small portions in fermentation samples and in green beer, because of|as a end result of} its reduction to acetoin is much faster than its formation. Bacteria, which can happen within the brewery as infections, are additionally likely to to|prone to} promote the formation of diacetyl. This can be achieved within the standard procedure by using a definite bunging over-pressure of zero. During storage, the beer must make clear by allowing the yeast and different haze-causing materials to settle, and its taste must refine and round-off. In order to achieve these necessities, the beer must be saved at zero to -2 �C for 1�2 weeks. Frequently, fermentation, maturation and conditioning take place in the identical vessel (one-tank process). Continuous fermentation or maturation methods use through-flow or tributary-flow methods or a bioreactor; nonetheless, these methods are rarely present in large manufacturing services. Transfer and yeast crop are indicated by large downward and small upward arrows, respectively. Pitching is performed at 6�7 �C, then the temperature is allowed to rise to 8�9 �C. In this time the extract is fermented fully and the diacetyl is lowered under the human threshold. For maturation, the maximum fermenting temperature is maintained until diacetyl is under zero. As recognized from experiments, the formation of byproducts is then elevated and the character of the beer changed to a more yeasty flavor. When as well as} the attenuation limit is reached, the beer is cooled down and lagered cold for 1 week. The desired counter-pressure is then saved minimal of|no less than} until to the tip of the maturation section. In the case of cold fermentation with integrated maturation, fermentation is performed at 8�9 �C to an attenuation degree of about 50%, then the refrigeration is switched off and temperature control set at 12�13 �C. At this temperature diacetyl elimination and final attenuation are reached, then the method proceeds as traditional. The elimination of the primary fermentation yeast by centrifuging and the addition of 10% kr�usen with an obvious degree of attenuation of 20�30% is practiced initially of the maturation section. Yeast in a continuing good situation can be cropped � as talked about � up to as} reaching of the ultimate degree of attenuation earlier than cooling the beer -1 �C. In no case may yeast be aerated, because of|as a end result of} then it might metabolize its reserve fund, and is ready to|this may} weaken and autolyse the yeast. It should be potential to use it for eight to 10 cold fermentations or four to five heat fermentations. Several items of course of equipment permit for yields up to as} 50% of the included beer. Afterwards, the yeast has a consistency of 18�22% dry matter and can be diluted with water to enable pumping. The quantity of recovered beer (%) can be calculated as: [(100 � dry matter earlier than recovery) � 100]/dry matter after recovering. The quality of recovered beer is dependent upon by} the equipment used and the situation of the yeast. Recovered beer has a slightly higher pH worth, more protein and nucleic acid constituents, and a yeasty and fruity smell/taste. Its extract of unique wort varies in accordance with the local laws (tax classification) from 7 to 14%. Bottom-fermented beer with an extract of unique wort of 10�14% includes a very large number of beer varieties, including pale and darkish beers, export beers (more than 12% extract of unique wort), M�rzen beers, and particular beers and festival beers (13�14% extract of unique wort). Within these limits there are such totally different beer varieties as Pilsener, Dortmunder, Munich, nicely as|in addition to} smokyflavor beer and cellar beers; these are, nonetheless, restricted to certain localities. The higher gravity limits for particular beers differ from nation to nation between thirteen and 15%. Top-fermented beers differ from bottom-fermented beers of their ingredients (more than 50% wheat malt or different malted cereals) and by their particular aroma, which is primarily induced by the top-fermenting yeast strains of S. Owing to the fast rate at which fermentation proceeds, a comparatively low pH worth of 4.
Generic arimidex 1mg
Another chance could be the late addition to the final rinsing water fed into the blending vessel. Furthermore, constituents in flavors and essences can lead to turbidity or precipitation depending on the beer used. This needs to be examined in superior throughout improvement of the formulation and presumably adjusted [5]. Moreover, turbid juices characterize a microbiological threat, since yeasts can develop in them very well. Analogous to the analysis of beer, the next are determined: � � � Original extract. Analogous to the analysis of beer, the next are additionally measured: � � � � Color. It is advisable to have these sorts of sensory assessments performed by an expert institute in the type of a shopper or even professional panel [1]. In the frame of the sensory analysis, odor, taste, carbonation taste and harmony are assessed on a five-point scheme. If one facet is given three points or less, a description of the off-flavor is important. An unbalanced bitterness results in a disharmonic total impression of the mixed beverage. During an evaluation it thus needs to be thought of that the one elements are evaluated with none flaws. Flavor, fruit taste, sweet/sour ratio, physique, taste improvement and off-flavor must be thought of. The most necessary attributes for the evaluation of the non-alcoholic elements are: � � � � � � Typical in taste and color. Chemical name of the odor/taste substances Citric acid Acetaldehyde Description of the odor/taste Possible causes bitter unripe apple, pungent, fruity malfunction throughout addition carbonic acid, fermentation onset of the beverage manufacturing water, contact with iron surfaces carbonic acid, hydrogen sulfide-forming microorganisms carbonic acid Iron metallic, rusty water, ink-like, iron-like, blood like rotten, rotten eggs, like fecal, sulfurous, like a stink bomb Hydrosulfide Ethyl mercaptan Drain link odor, rain gutter drain like, leek-like, like rotten greens vinegar-pungent, bitter, fermented, pungent earthy, damp soil, humus-like Acetic acid starting fermentation of the beverage manufacturing water, microorganisms Ethyl fenchol 2,6-dichlorophenol pharmacy/medicine taste, like dentist, phenolic, like hospital cork note, moldy, musty, like a moist basement manufacturing water, detergent residues, bottle cleansing machine Trichloroanisole 268 11 Beer-Based Mixed Drinks and thus be sensitized for the incidence of those flaws. Furthermore, possible causes are talked about for the incidence of off-flavor notes. In badly cleaned niches, lactic acid micro organism can adapt to model new} medium possessing a lower pH than beer and this presents a fantastic threat. Due to carbonization, cardio microorganisms have hardly any probability to develop even in beer-based mixed drinks. Different microbiological threat potentials come up in the ready drink depending on the beverage formulation [2]: � � Beer + sugar + acid + essence. Without any fermentable carbohydrates only lactic acid micro organism play a role in this medium. Attention ought to be paid to microbiologicals belonging to the next categories [7]: � � � � Obligate. A sure focus of vitamin C will get lost even during the storage phase due to of} oxygen inside the packaging and dissolved in the product. Higher additions are thus needed, which must be determined in superior in stability and cargo exams. Effective conservation of sugar-sweetened beer-based mixed drinks with preserving agents in all probability not|will not be} adequate and ought to be verified precisely. The conservation of the uncooked material is advisable because the that} microbiological stability can simply be ensured throughout fractional removal in the bottling factory. The most effective technique for stabilizing the final beverage is generally pasteurization of the crammed bottles. The temperature/time frame are coordinated and differ for the respective beverages, similar to beer, beer-based mixed drinks or delicate drinks. The determined and coordinated temperature/time frame can be taken from Figure 11. In cost processes it is recommended to put in the syrup for the drink first and then add the beer. A homogenous mixing ought to be aimed for throughout syrup manufacturing to avoid local pH gradients. As talked about above, a well-established order of the elements needs to be chosen throughout in-line mixing in order to to} avoid pH shocks for beer constituents. Additionally, it ought to be identified that even a too high pH value can cause a adverse impact [1]. In addition, a sharp filtration is important in order that no yeasts get into the sugar-sweetened ready drinks particularly [5]. The protein focus of the beer has a direct affect on possible turbidity after mixing with the basic elements or flavors. Thus, clear physicochemical boundaries are set [5] and not all mixing choices can merely be carried out for every beer. However, this will already 272 11 Beer-Based Mixed Drinks be investigated in stability exams carried out by the supplier throughout formulation preparation. On the other hand, beer has additionally a potential to kind these sorts of turbidities in the ultimate beverage. The brewery needs to use sharp filtered and stabilized beers to be able to|be succesful of|have the flexibility to} use the number of new beverage sorts [1]. The merchandise are well protected against oxidation by pre-evacuation and pre-loading of the bottles. The modified microbiological sensitivity, particularly for those sweetened with sugar, is again necessary in the context of beer-based mixed drinks. Thus, in order to to} avoid secondary infections [2] consideration ought to especially be paid in the direction of|in direction of} hygiene in the bottle washer and in the filling area. Since mixed beverages more and more characterize modern beverages, the mixtures are doubtless to|prone to} be crammed in white glass bottles. Light-induced oxidation reactions are extraordinarily high in white glass; however, these can be suppressed by masking the bottles. Even in glass bottles the flavors of the delicate drink are strongly affected besides the classical oxidation reactions of the beer constituents. A know reaction is, for instance, the breakdown of citral to cresol which causes an off-flavor. Apart from the lack of taste, oxidative-induced lightening of the color is an issue which, as already talked about, can be prevented by the addition of antioxidants. It is necessary to know the affect of the packaging on the product quality, since altering packing. In addition to the talked about aspects, it needs to be thought of that new mixture compositions, particularly for exotic variants, can cause issues. In order to avoid undesirable precipitation reactions the formulation needs to be individually checked with respect to mixing order and hot spots due to of} high concentrations of single constituents [1]. Mixing of the elements: (i) preparation of the syrup for the delicate drink fraction and the blending of beer in a cost course of or in-line mixing, or (ii) steady in-line mixing; Carbonization/impregnation. Fruchtsaft- und Limonadenbetriebe, Wasser, Betriebshygiene, Milch und Molkereiprodukte, Begleitorganismen der Getr�nkeindustrie, Fachverlag Hans Carl, N�rnberg. For filling beer, there are 4 primary categories of packaging in use worldwide: � � � � Glass bottles. In the case of a returnable concept, goes to be|will in all probability be} essential to clarify whether the container can be cleaned with out forsaking any residues. In addition, although extra from a advertising viewpoint, the load and the reclosability of the container are additionally necessary. Glass bottles excel in terms of|when it comes to|by method of} high mechanical power when exposed to mechanical stresses and axial influences. For inside strain resistance, this specification calls for a minimal value of 10 bar for nonreturnable bottles and not lower than 12 bar for overseas-export non-returnables (with a quantity of more than 0. The responsibility inside strain resistance for returnable bottles is specified as not lower than 10 bar. In addition, the specification defines an impression resistance of greater than 35 ips for non-returnable bottles, of greater than 50 ips for overseas-export bottles and of greater than 60 ips for returnable bottles. This barrier property is particularly necessary in the case of beer, by cause of its tendency to oxidation reactions by some of its constituents following a lengthy interval of storage. By cause of its high mechanical power and resistance to chemical compounds, glass is ideally suited to returnable applications. Mineral-water and beer bottles in a returnables circuit, for example, achieve as much as} forty turnarounds. For sure markets, the thermal and dimensional stability of glass is an extra significant factor; because the that} crammed bottles can be pasteurized at temperatures above 60 �C, avoiding any substantial and abrupt temperature adjustments, thus enabling shelf-life to be extended.
Arimidex 1 mg
The degradation results from the activity of a ribonuclease encoded by the S locus. The ribonuclease is secreted from the cells of the type within the extracellular matrix, which lies alongside the growing pollen tube. In summary, self-incompatibility is a mechanism that prevents self-fertilization plenty of} flowering plant species. The working of this self-incompatibility mechanism has essential penalties for plant breeders because of|as a outcome of} it inhibits the production of inbred and hybrid vegetation. Pollination by Insects Bees are maybe the most important pollinator of many garden vegetation and most industrial fruit bushes (Figure 32. Bees acquire energy-rich pollen or nectar for his or her survival and energy this OpenStax guide is on the market for free at cnx. They visit flowers that are be} open during the day, are brightly colored, have a strong aroma or scent, and have a tubular shape, usually with the presence of a nectar information. A nectar information contains regions on the flower petals that are be} visible solely to bees, and not to people; it helps to information bees to the middle of the flower, thus making the pollination process extra efficient. Recently, there have been many reports concerning the declining inhabitants of honeybees. These flowers, which produce nectar, normally have uninteresting colours, corresponding to brown or purple. They are found on the corpse flower or voodoo lily (Amorphophallus), dragon arum (Dracunculus), and carrion flower (Stapleia, Rafflesia). Butterflies, such as the monarch, pollinate many garden flowers and wildflowers, which normally occur in clusters. These flowers are brightly colored, have a strong fragrance, are open during the day, and have nectar guides to make entry to nectar easier. The flowers pollinated by moths are pale or white and are flat, enabling the moths to land. One well-studied instance of a moth-pollinated plant is the yucca plant, which is pollinated by the yucca moth. The shape of the flower and moth have adapted in such a method as to enable profitable pollination. As the eggs develop into larvae, they acquire food from the flower and growing seeds. Thus, both the insect and flower benefit from each other in this symbiotic relationship. The flowers are normally large and white or pale-colored; thus, they are often distinguished from the darkish surroundings at night time. The flowers have a strong, fruity, or musky fragrance and produce large quantities of nectar. As the bats seek the nectar, their faces and heads become lined with pollen, which is then transferred to the subsequent flower. Pollination by Birds Many species of small birds, such as the hummingbird (Figure 32. Flowers visited by birds are normally sturdy and are oriented in such a method as to enable the birds to stay near the flower without getting their wings entangled within the close by flowers. Brightly colored, odorless flowers that are be} open during the day are pollinated by birds. Botanists have been recognized to determine the vary of extinct vegetation by accumulating and figuring out pollen from 200-year-old bird specimens from the identical site. Pine cones are brown and unscented, while the flowers of wind-pollinated angiosperm species are normally green, small, might have small or no petals, and produce large quantities of pollen. In wind-pollinated species, the microsporangia hang around of the flower, and, as the wind blows, the lightweight pollen is carried with it (Figure 32. Note how both constructions are mild and feathery to higher disperse and catch the wind-blown pollen. Pollination by Water Some weeds, corresponding to Australian sea grass and pond weeds, are pollinated by water. They grow in a variety of specific habitats, primarily within the tropics of Asia, South America, and Central America. They use a way food deception, by which shiny colours and perfumes are provided, but no food. Anacamptis morio, generally the green-winged orchid, bears shiny purple flowers and emits a strong scent. The bumblebee, its main pollinator, is interested in the flower due to the strong scent-which normally indicates food for a bee-and within the process, picks up the pollen to be transported to another flower. Chiloglottis trapeziformis emits a compound that smells the identical as the pheromone emitted by a feminine wasp to appeal to male wasps. The male wasp is interested in the scent, lands on the orchid flower, and within the process, transfers pollen. Some orchids, like the Australian hammer orchid, use scent as well as|in addition to} visible trickery in yet another sexual deception strategy to appeal to wasps. The flower of this orchid mimics the appearance of a feminine wasp and emits a pheromone. The male wasp tries to mate with what appears to be a feminine wasp, and within the process, picks up pollen, which it then transfers to the subsequent counterfeit mate. Double Fertilization After pollen is deposited on the stigma, it should germinate and grow by way of the type to attain the ovule. The microspores, or the pollen, comprise two cells: the pollen tube cell and the generative cell. The pollen tube cell grows right into a pollen tube by way of which the generative cell travels. The germination of the pollen tube requires water, oxygen, and sure chemical signals. In the meantime, if the generative cell has not already cut up into two cells, it now divides to form two sperm cells. The pollen tube is guided by the chemical substances secreted by the synergids present within the embryo sac, and it enters the ovule sac by way of the micropyle. Of the two sperm cells, one sperm fertilizes the egg cell, forming a diploid zygote; the other sperm fuses with the two polar nuclei, forming a triploid cell that develops into the endosperm. Together, these two fertilization this OpenStax guide is on the market for free at cnx. The fertilized ovule varieties the seed, whereas the tissues of the ovary become the fruit, normally enveloping the seed. After fertilization, the zygote divides to form two cells: the higher cell, or terminal cell, and the decrease, or basal, cell. The division of the basal cell gives rise to the suspensor, which finally makes reference to the maternal tissue. The suspensor supplies a route for vitamin to be transported from the mother plant to the growing embryo. The terminal cell also divides, giving rise to a globular-shaped proembryo (Figure 32. In dicots (eudicots), the growing embryo has a coronary heart shape, the presence of the two rudimentary cotyledons (Figure 32. In non-endospermic dicots, corresponding to Capsella bursa, the endosperm develops initially, but is then digested, and the food reserves are moved into the two cotyledons. As the embryo and cotyledons enlarge, they run out of room contained in the growing seed, and are pressured to bend (Figure 32. Embryonic development is suspended after some time, and development is resumed solely when the seed germinates. The growing seedling will depend on the food reserves stored within the cotyledons till the primary set of leaves begin photosynthesis. After fertilization, the zygote divides to form an higher terminal cell and a decrease basal cell. Wise; scale-bar knowledge from Matt Russell) Development of the Seed the mature ovule develops into the seed. A typical seed incorporates a seed coat, cotyledons, endosperm, and a single embryo (Figure 32. Monocots, corresponding to corn (right), have one cotyledon, known as the scutellum; it channels vitamin to the growing embryo. Both monocot and dicot embryos have a plumule that varieties the leaves, a hypocotyl that varieties the stem, and a radicle that varieties the root.
Quality arimidex 1mg
Consequently, a longer adjustment period throughout demanganization is to be expected as comparability with} the deferrization filter. Two separated filter items are usually advantageous for the elimination of iron and manganese. Deferrization happens within the first filter (saving cleaning water, iron compounds are easily rinsed off). In the second filter demanganization is carried out (increased holding time, gradual coarsening of the filter materials by way of black manganese dioxide). There, contaminants adsorb onto the surface of the activated carbon and are thus fastened. In this repeatedly beneficial and used process, the problem is shifted as this alternative supplies no elimination and mineralization (see Section 4. The activated-carbon filter serves, amongst other issues, for the elimination of: � � � � � � Odorous substances and flavorings. In order to enable sterilization of the filters and thus avoid microbiological contamination, the activated-carbon filter ought to be made out of chrome steel 4. Apart from the change in hardness former (softening), the reduction of nitrate focus within the brew water plays an everincreasing role. Through the addition of saturated lime water, the calcium focus and at enough basicity (pH > 10) even the magnesium focus are lowered. The added lime slurry is quantitatively deposited as calcium carbonate or magnesium hydroxide. This has no influence on the mash pH because the buffering capability of the water is low. The m value serves to determine the softening impact; the p value offers an indication of the right dosage of lime. The hardness former of the water (calcium, magnesium, sodium) may be eliminated by using weak and/or sturdy acidic ion-exchange resins. In the subsequent use of weak basic resins, the water may be utterly demineralized. Nitrate-selective (strong basic) resins, the opposite hand|however|then again}, only reduce the nitrate focus. Ion exchangers are strong supplies may be} place to} adsorb cations and anions from an electrolytic resolution, and exchange these with an equivalent of quantity ions 114 4 Brew Water of the identical charge. In a cation exchanger, sulfonic acid residues are fastened within the matrix of the resin. Cations, corresponding to calcium ions, enter with out problem; anions, corresponding to chloride ions, are repulsed by the negatively connected ions. Only legally licensed regenerating supplies are allowed to be used to regenerate the ion exchanger. Furthermore, the resins must be safe for all foods � no flavorful, olfactorial or health-damaging supplies are allowed to be given off. The following factors ought to be thought of for the planning of an ion-exchanger plant: � � � � � Free from floating supplies. A widespread process combination of a cation and anion exchanger is presented in Figure 4. The optimum following anion exchanger may be designed either to serve as a weak basic resin for full demineralization or as a powerful basic resin to selectively take away nitrate. Depending on the selection of the mixture processes, a particular regeneration scheme is applied. This must be larger than the osmotic strain, in order that the water molecules are forced to migrate by way of a semipermeable membrane, whereas the salts are mostly retained. For a greater overview, the brewery-relevant processes and needed definitions are defined by referring to Figure 4. In reversed osmosis the concentrate is the concentrated water that, similar to the volume of obtained permeate, incorporates the dissolved substances of the feed in concentrated form. The yield is the speed of permeate volume to the feed volume: yield (%) = permeate/crude water � 100%. It is important to notice that with rising yield the salt focus within the permeate additionally increases. An optimum between quality of the permeate and the yield of the permeate must be established for every use. The Bavarian Purity Law (Reinheitsgebot), one of the oldest restrictions on food, which regulates German beer production, was additionally proclaimed without any knowledge of yeast. First progress was made with the event of the microscope by Antonie van Leeuwenhoek (1652�1725) [2]. Gay-Lussac arrange the first full formula for the fermentation of sugar; however, he nonetheless believed in fermentation as an oxidative process [3]. An important advance was made in 1837 by Theodor Schwan by way of his experiments involving sterilized grape juice. Under the microscope he discovered small proliferating items and concluded that these things have been living organisms. Although the evidence for the relationship between yeast and fermentation had been discovered, leading chemists at the moment, like Liebig and W�hler, nonetheless criticized these results. The emerging brewing business within the nineteenth century already knew in regards to the importance of yeast and many of|and lots of} brew masters who arrange new businesses brought along their very own yeast. The first differentiations between top- and bottomfermenting strains have been launched. Between 1855 and 1876, Luis Pasteur revealed his fermentation theory and distinguished between aerobic and anaerobic utilization of sugars by yeast [5]. It took almost 100 years to elucidate the whole biochemical pathways of fermentation. Again, it was Louis Pasteur who discovered the production of fermentation byproducts (glycerol) in 1860. Arthur Harden and William Young showed that phosphate is required for glucose fermentation. The importance and destiny of pyruvate was examined by Neubauer, Fromherz and Neuberg in 1911. A variety of scientists have been involved, corresponding to Embden, Meyerhof, Parnas and Warburg. They discovered important reactions, the influence of coenzymes, leading finally to the enlightenment of the whole fermentation pathway [6]. The impact describes a limited respiratory exercise in yeast within the presence of a certain sugar focus. Probably an important steps in yeast research concerning the brewing business have been made by Hansen. In 1883, he cultivated the first pure cultures of brewing yeast and carried out them in breweries. Hansen adapted methods invented by the famous doctor Robert Koch, isolating single cells on plate agar cultures. His work was the first that allowed working with pure cultures, not only free from beer spoilage micro organism, but additionally void of wild yeasts [8]. Hansen and Lindner additionally took great care about transferring the isolated cultures from the laboratory into the brewery. Both developed easy propagation gadgets to be able to} grow larger yeast quantities and pitch larger fermentation vessels. In 1913, Coblitz and Stockhausen launched a method of rising yeast by transferring it through the high kr�usen stage [10]. The chance of contemporary molecular biology has offered major insights into the yeast cell. In addition, yeast has become the model microorganism in cell research and its genome was the first of any eukaryotic cell to be utterly decoded. Two types of Saccharomyces yeasts are involved in beer fermentation, top-fermenting (or ale) yeasts and bottom-fermenting (or lager) yeasts. Both yeast species belong to the carefully associated Saccharomyces sensu stricto species [13].
Order 1 mg arimidex
The formation of acetylides and alkynides (alkynyl Grignard reagent and alkylnyllithium) are necessary reactions of terminal alkynes (see Section four. Acetylides and alkynides bear nucleophilic addition with aldehydes and ketones to produce alcohols (see Section 5. H3O+ O R C Y Y = H or R Aldehyde or ketone They react with alkyl halides to give internal alkynes (see Section 5. Any terminal alkyne can be transformed to acetylide and alkynide, after which alkylated by the reaction with alkyl halide to produce an internal alkyne. In these reactions, the triple bonds are available for electrophilic additions to quantity of|numerous|a selection of} other useful teams. Therefore, to be able to} perceive the chemical nature, physical properties, stability, pharmacological actions and toxicities of a majority of drug molecules, the data of fragrant chemistry is extremely necessary. Before we look into the precise examples of varied medication that belong to this fragrant class, let us attempt to perceive what aromaticity actually is. However, there are a selection of other nonbenezenoid compounds that can be be} categorised as fragrant compounds. He isolated benzene from a compressed illuminating fuel that had been made by pyrolysing whale oil. In 1834, Eilhardt Mitscherlich synthesized benzene by heating benzoic acid with calcium oxide. In the above construction of benzene, there are three double bonds and 6 p electrons, and it � is a planar molecule. Macrocyclic these are monocyclic nonbenzene constructions, and the ring sizes are quite huge. N Pyridine N H Pyrrole Pyridine has p electron construction similar to that of benzene. Each of the 5 sp2-hybridized carbons has a p orbital perpendicular to the airplane of the ring. N: Structure of pydridine with p orbitals the scenario in pyrrole is slightly different. It has 4 sp2-hybridized carbons, each of which has a p orbital perpendicular to the ring and contributes one p electron. The nitrogen atom can also be|can be} sp2-hybridized and its lone pair electrons occupies a p orbital. Just quantity of} examples of pharmaceutically necessary fragrant compounds are cited here. Aspirin, a well known non-narcotic analgesic and antipyretic drug, is a classic example of a pharmaceutically necessary benzene by-product. Aromatic compound valium is prescribed as a tranquillizer and ibuprofen as an anti-inflammatory, and sulpha medication. Taxol, probably the greatest selling anticancer medication of contemporary time, additionally belongs to the category of fragrant compounds. According to his proposals, in benzene (a) all six carbon atoms are in a ring; (b) all carbon atoms are bonded to one another by alternating single and double bonds; (c) one hydrogen atom is hooked up to each carbon atom; (d) all hydrogen atoms are equivalent. H H H H Kekul� construction of benzene H H � � Limitations of Kekule construction the Kekule construction predicts that there ought to be two different 1,2-dibromobenzenes. Later, this proposal was proved to be incorrect, end result of|as a outcome of} no such equilibrium exists! The resonance explanation of the construction of benzene the resonance concept can be utilized successfully to clarify the construction of benzene. It has � actually been confirmed that benzene is a planar molecule, and all of its C� C �). Thus, as a substitute of drawing the benzene construction utilizing different single and double bonds, a hybrid construction can be drawn as follows. Hybrid construction of benzene the hybrid construction of benzene is represented by inscribing a circle within the hexagon as depicted above. With benzene, the circle represents the six electrons would possibly be} delocalized in regards to the six carbon atoms of the benzene ring. The resonance concept accounts for the much higher stability of benzene (resonance energy) in comparison with the hypothetical 1,three,5-cyclohexatriene. The molecular orbital explanation of the construction of benzene the bond angles of the carbon atoms in benzene are one hundred twenty. All carbon atoms are sp2-hybridized, and every carbon atom has a single unhybridized p orbital perpendicular to the airplane of the ring. The carbon sp2-hybridized orbitals � overlap to type the ring of the benzene molecule. H + H H + + + + H + H H Doughnut-shaped cloud of electrons Six p atomic orbitals, one from each carbon of the benzene ring, mix to type six p molecular orbitals. Three of the molecular orbitals have energies lower than that of an isolated p orbital, and are generally known as|often identified as} bonding molecular orbitals. Another three of the molecular orbitals have energies higher than that of an isolated p orbital and are called antibonding molecular orbitals. Stability of benzene Benzene has a closed bonding shell of delocalized p electrons. Therefore, within the case of cyclohexadiene, the place there are two double bonds, the power required for the hydrogenation can be calculated as 2 � �28:6 � �57:2 kcal/mol. In follow, the experimental worth kind of|is sort of} close to this calculated worth, and is �55. Many benzene derivatives have trivial names, which may present no resemblance to the name of the hooked up substituent group. The three possible isomers of a disubstituted benzene are differentiated means of} the names ortho, meta and para, abbreviated as o-, m- and p-, respectively. Br Br Br Br ortho-Dibromobenzene meta-Dibromobenzene Br Br para-Dibromobenzene If the 2 teams are different, and neither is a group that offers a trivial name to the molecule, the 2 teams are named successively, and the word four. If one of the two teams is the kind that offers a trivial name to the molecule, then the compound as a by-product of this compound. If the teams are the identical, each is given a number, the sequence being the one that offers the lowest combination of numbers; if the teams are different, then the last-named group is understood to be in position 1, and the other numbers conform to this. If one of the teams that give a trivial name is current, then the compound as having the particular group in position 1. Before we go into any particulars of such reactions, let us attempt to perceive the following phrases. An fragrant hydrocarbon with a hydrogen atom removed is � called an aryl group, designated by Ar�. The benzene ring with one hydrogen atom removed � � (C6H5�) the phenyl group, designated by Ph�. An electrophile (E�) reacts with the benzene ring and substitutes for certainly one of its six hydrogen atoms. Electrophiles assault the p system of benzene to type a delocalized nonaromatic carbocation (arenium ion or s complex). Some particular examples of electrophilic substitution reactions of benzene are summarized under (see Chapter 5). A summary of those results of substituents on reactivity and orientation of electrophilic substitution of substituted benzene is introduced under. The relative price of � reaction decided by} whether or not the substituent group (� S) withdraws or � releases electrons relative to hydrogen. When � S is an electron-releasing group the reaction is quicker, whereas when this group is an electron-withdrawing group a slower price of reaction is noticed. S S + E+ + -S releases electron Transition state is stabilized + + + + S E H E H Reaction is quicker Arenium ion is stabilized S + E+ -S withdraws electron Transition state is destabilized + + S + + + S E H E H Reaction is slower Arenium ion is destabilized Orientation Similarly, teams already current on the benzene ring direct the orientation of model new} substituent to ortho, para or meta positions. For example, nitration of chlorobenzene yields ortho-nitrochlorobenzene (30%) and para-nitrochlorobenzene (70%). When the substituent (� S) bonded to a benzene ring is a more electronegative atom (or group) than carbon;. As a consequence, an electrophilic assault will be less favoured due to an extra full positive cost on the ring. Inductive electron donation makes the ring more reactive course of|in path of} electrophilic substitution due to the increased availability of electrons. The presence of a substituent may improve or lower the resonance stabilization of the intermediate arenium ion complicated.
References:
- https://www.lacare.org/sites/default/files/AsthmaToolkit_02192015.pdf
- https://www.madinamerica.com/wp-content/uploads/2015/08/DSM-II.pdf
- https://ictr.johnshopkins.edu/wp-content/uploads/2013/07/7.19.13.Miller-Clinical-Trials.pdf
.png)